Rev Med UAS
Vol. 12: No. 1. Enero-Marzo 2022
ISSN 2007-8013
Hector Navarro-Chavez, MD1, Julio Garza-Vega, MD1, Victor Segura-Ibarra, MD, PhD2,3,Marisa G. Frausto-Alejos, MD2, Eduardo Flores-Villalba, MD, MSc1,2,3,*
*Autor de correspondencia:Dr. Eduardo Flores-Villalba
Escuela de Medicina y Ciencias de la Salud TEC de Monterrey,
Monterrey 64849, México. Phone: +52 8358-2000 Ext. 5126
Email: eduardofloresvillalba@tec.mx
DOI http://dx.doi.org/10.28960/revmeduas.2007-8013.v12.n1.007
Texto Completo PDFRecibido 29 de junio 2021, aceptado 06 de julio 2021
ABSTRACT
Neuroendocrine tumors are a vast group of neoplasms. Insulinomas are the most frequent functioning pancreatic neuroendocrine tumors. Therapeutic gold standard is surgery without which most patients have a fatal outcome due to hypoglycemia. Herein we report a case of a 91-year-old female, with a 3 month history of asthenia and adynamia, which progressed to several neuroglycopenic events, leading to hospitalization. An abdominal CT-scan reported 1.8 x 1.4 cm pancreatic tumor. Labs reported proinsulin 40.5 pM/L. Laparoscopic enucleation was performed. 3 months later she presented a pancreatic pseudocyst treated successfully by endoscopy. Insulinomas, are considered to have low-malignant potential, it is a rare endocrinal tumor and there is minimal mention about a consensus for optimal surgical treatment. Insulinoma remains an exclusion diagnosis after analyzing other differentials. Correct utilization of pre-operative imaging is critical in pre-surgical planning to choose the appropriate surgery.
Keywords: Laparoscopy; Insulinoma; Whipple’s triad; Neuroendocrine tumor.
RESUMEN
Los tumores neuroendocrinos son un extenso grupo de neoplasias, siendo el insulinoma el tumor neuroendocrino pancreático funcionante más frecuente. El estándar de oro terapéutico es quirúrgico, sin el cual la mayoría de pacientes obtiene un desenlace fatal por hipoglucemia. Reportamos el caso de una femenina de 91 años, con 3 meses de evolución de astenia y adinamia, que progresó a eventos neuroglucopénicos, y posterior hospitalización. El TC abdominal reportó un tumor pancreático de 1.8 x 1.4 cm. Los laboratorios reportaron proinsulina 40.5 pM/L. Se realizó enucleación laparoscópica. 3 meses después presentó un pseudoquiste pancreático, tratado satisfactoriamente mediante endoscopia. El insulinoma es considerado de bajo potencial maligno, es un tumor endocrino raro y hay poca información sobre un consenso para el tratamiento quirúrgico óptimo. Es un diagnóstico de exclusión después de analizar otros diferenciales. El uso correcto de imágenes preoperatorias es fundamental en la planeación y elección de la cirugía apropiada.
Palabras Clave: Laparoscopia; Insulinoma; Triada de Whipple; Tumor neuroendocrino.
Introduction
Neuroendocrine tumors are a vast group of neoplasm, which can origin from many organs throughout body. Though they are usually located in the abdominal cavity and lungs. Despite considered to be a rare entity, overall, current incidence is estimated to be up to 5.25 cases per 100,000 in general population.1 Whereas pancreatic neuroendocrine tumors (PanNETs), being insulinoma the most frequent functional tumor has an incidence of 4 per million per year, which is less when compared to the incidence of adenocarcinomas.2,3 Given its frequency, diagnosis is sometime overviewed and tends to be an exclusion diagnosis. It typically affects older individuals, with a higher tendency on women, with a median age of diagnosis on the 5th decade of life.3,4
In accordance to the SCARE criteria, herein we report an insulinoma treated through laparoscopic intervention, aiming to provide additional information to current literature regarding our management.
Presentation of case
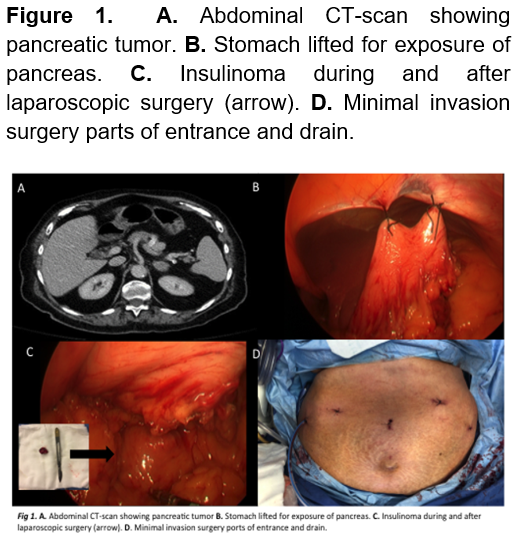
This 91-year-old female, with a past medical history of hypertension, presented with recurrent hospitalizations due to asthenia, adynamia, and syncope which progressed to several neuroglycopenic events that responded to glucose administration. Family history is positive for a maternal, unspecified tumor. The patient underwent screening for a probable neuroendocrine tumor, reporting the following labs: TSH 2.63 mIU/L, Free T4 0.78 mcg/dL, total T4 6.8 mcg/dL, Urea 66 mg/dL, Cr 1.4 mg/dL, normal LFTs, Na 135 mEq/L, K 4.5 mEq/L, Cl 100 mEq/L, catecholamines (urine) epinephrine 5 mcg/day, norepinephrine 32 mcg/day, dopamine 78 mcg/day, proinsulin 40.5 pmol/L, serum insulin 23 pmol/L, C-peptide 4 ng/mL, serum cortisol 11.79 mcg/dL. An abdominal CT-scan was performed which reported pancreatic fat infiltration within an heterogeneous, oval mass with defined edges, isodense to parenchyma of 43 HU that enhances up to 115 HU with contrast, and measures 1.8 x 1.4 cm, compatible with functional tumor adjacent to the body (Figure 1). Integrating such findings, the diagnosis of an insulinoma was achieved.

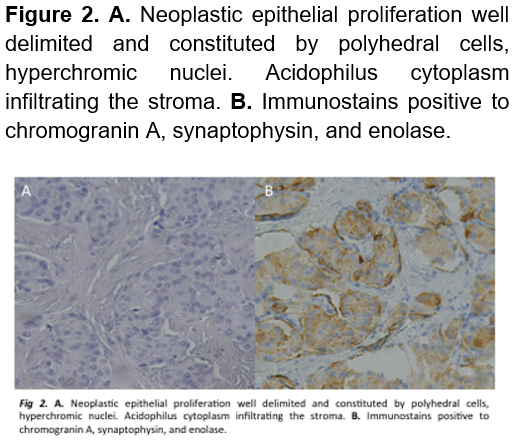
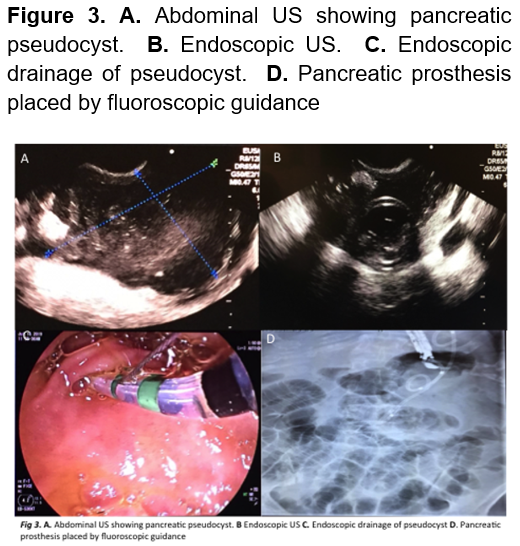
A laparoscopic enucleation was performed, elevating the stomach to obtain a clear field of view revealed a tumor of approximately 2 x 1.5 cm (Figure 1). Drainage was removed and she was discharged 5 days after, without a single hypoglycemic event. Pathology confirmed a neuroendocrine tumor compatible with insulinoma. (Figure 2). Three-months later, the patient returned to the ER presenting abdominal pain, her initial labs only reported leukocytosis. A CT scan was performed which remarked a 7 x 8 cm retroperitoneal hypodense mass compatible with a pancreatic pseudocyst. Endoscopic drainage was performed, and she was discharged a day later without a single complication (Figure 3). 1 year after the surgery, patient is healthy and without any hypoglycemic event.
Discussion
PanNETs are uncommon neuroendocrine neoplasms, which are often divided into functional tumors (those that produce endocrine hormones and tend to clinical syndromes) and non-functional (no clinical syndrome associated to endocrine hormones). Functional PanNETs can produce hormones such as insulin, glucagon, vasoactive intestinal peptide (VIP), somatostatin, pancreatic polypeptide (PP), and others.
Functional tumors account only for 10% of PanNETs, while the rest non-functional will cause clinical symptoms associated to compression.4,6 Up to 10% of insulinomas can be a part of multiple endocrine neoplasia type 1 (MEN1) syndrome. 7 This last condition is more frequent in men and tend to have an earlier onset of manifestation with this association.8,9
From all functional PanNETs, insulinoma is the most common neoplasm of this group and cause of endogenous hypoglycemia in non-diabetic patients, fortunately, more than 90% of these tumors are benign.2 The clinical manifestations of insulinoma is with Whipple’s triad: low blood glucose (<40-50 mg/dL), symptoms of hypoglycemia, and relief of symptoms after glucose administration.10 The clinical diagnosis is accurate with the patient presented on the case.
The median duration of symptoms before diagnosis was less than 1.5 years. However, some patients could had been symptomatic for decades, and approximately 20% of patients were misdiagnosed with a neurologic or psychiatric disorder before the diagnosis of insulinoma was made.11
The actual gold standard for the diagnosis of insulinoma is a 48-hour fast with negative levels for sulfonylurea and beta-hydroxybutyrate while using a pinpoint of discrimination for proinsulin ≥ 22 pmol/L.12 Followed by an imaging modality in which most cases is a multiphasic CT scan, even though endoscopic ultrasound is more sensitive. Though CT scan is preferred since is not invasive and doesn’t require an expert endoscopist. In some cases, radiologic diagnosis can be a real challenge due to tumor’s size.13 As stated on the presentation of case, it also fulfills laboratory and imaging criteria, with a proinsulin of 40.5 pmol/L and an image compatible with functional tumor. In cases with doubt of localization, exploratory surgery with manual palpation is often successful though, if still doubt, the patient should go under more evaluation before doing a definitive intervention.14 The final diagnostic will always be through pathology report.
The diagnosis of malignancy is only defined out in the presence of metastasis but there is recommended a special approach to the patients with clinical history of less than 6 months; fasting time before hypoglycemia less than 8 h; insulin and C-peptide concentration >25 U/mL and >4 ng/dL at the glucose nadir, respectively; and tumor size larger than 2.5 cm. Very high levels of chromogranin A and pro insulin may also indicate greater potential for aggressive clinical behavior.15
The therapeutically gold standard is surgery. Insulinomas lean towards having a small size (82% < 2cm, 47% < 1cm) and don’t tend to metastasize, that’s why enucleation is frequently used on these tumors (Figure 1), the most frequent complications are a pancreatic fistula and stenosis of the main pancreatic duct. When enucleation isn’t possible a formal pancreatectomy (Whipple’s procedure or distal pancreatectomy, depending on tumor’s localization) and lymphadenectomy are preferred. 2,16
For patients who are not surgical candidates or those who are waiting for optimal conditions, several measures can be taken to manage the symptoms, including dietary modification and pharmacologic agents like diazoxide, the somatostatin analogues octreotide and lantreotide and verapamil. Data suggests that refractory cases may respond to treatment with everolimus.3
Pancreatic pseudocyst is not a typical complication, as stated earlier. Whereas the therapeutically approach depends on size and clinical manifestations, which can be conservative or some sort of invasive procedure. The most common procedure is the endoscopic drainage guided with ultrasound, this approach was chosen given that she was symptomatic. Success rates are of 70% with a morbidity of 19%.17
The use of minimally invasive surgery has a decrease of 7.9% and 4.7% on postoperative complications and mortality. 18 However, the benefits include faster recovery from postoperative pain, less scarring, and a quicker return to regular work-related activities.
Overall, insulinomas are mostly benign and the prognosis is based on clinical stage classification.19 In addition, in those who do not undergo a surgical treatment the main cause of fatal outcome is hypoglycemia.
Conclusion
Even though insulinoma is the most common functional PanNet it still is a rare entity in endocrine cancer pathology. Its size can be a challenge for radiologic diagnosis, and final pathology from the surgical specimen is required for an accurate diagnostic. There remains no consensus on the optimal surgical treatment for PanNETs, in part due to its rarity. Careful utilization of pre-operative imaging modalities is critical in pre-surgical planning to choose the appropriate surgery.
Declarations
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not - for - profit sectors.
Conflicts of interest: The authors declare that they have no competing interests.
Availability of data and material (data transparency): All data of the patient support the published claims. The data from the current study are available from the corresponding author on reasonable request.
Code availability (software application or custom code): Not applicable
Authors' contributions: All authors had access to the data and a role in writing this manuscript.
Ethics approval (include appropriate approvals or waivers): This case report has ethics approval and comply with the specific requirements of the institution.
Consent to participate (include appropriate statements): The patient has provided written consent to publish his case and details of his hospitalization, including imaging studies and gross pathology.
Consent for publication (include appropriate statements): All the authors and the patient agree for the publication of this article.
REFERENCIAS